RECOMMENDED
NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) recommend avapritinib (AYVAKIT) (if platelets ≥50 x 109/L) as an NCCN Category 2A, preferred first-line treatment option for advanced systemic mastocytosis (SM), including aggressive SM (ASM), SM with an associated hematologic neoplasm (SM-AHN) (when the SM component requires prioritization over the AHN component), and mast cell leukemia (MCL).2
NCCN=National Comprehensive Cancer Network.
(95% CI: 42%, 70%)*
Proven efficacy and demonstrated duration of response1
38.3-month median duration of response (DOR) demonstrated in clinical trials (95% CI: 19, NE) DRIVEN PRIMARILY by SM-AHN patients
EXPLORER and PATHFINDER were 2 multicenter, single-arm, open-label clinical trials evaluating the efficacy and safety of AYVAKIT for patients with Advanced SM.1
Efficacy was based on overall response rate (ORR) per modified IWG-MRT-ECNM criteria across all evaluable patients dosed up to 200 mg daily (N=53). In the subgroup of patients with MCL, the efficacy was based on complete remission (CR).1
53 patients were evaluable for a response, with a median follow-up of 11.6 months (95% CI: 9.9 to 16.3 months)1
Patients enrolled in EXPLORER received a starting dose of AYVAKIT ranging from 30 mg to 400 mg orally once daily. In PATHFINDER, patients were enrolled at the recommended starting dose of 200 mg orally once daily1†
*ORR per modified IWG-MRT-ECNM is defined as patients who achieved a CR, CRh, or PR.
†The recommended dosage for patients with Advanced SM is 200 mg orally once daily per the US Prescribing Information.
View criteria for response-evaluable patients1
View baseline demographic characteristics (N=53)1
Proven efficacy across all subtypes and regardless of prior antineoplastic therapy1,7
All subtypes: ASM, MCL, SM-AHN (N=53)
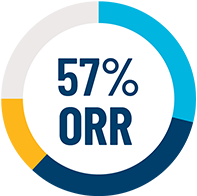
(95% CI: 42%, 70%)a
72% ORR was achieved with the addition of patients who had clinical improvementb
Median DOR of 38.3 months (95% CI: 38, NE)
- Median time to response (n=30)7: 2.1 months
- Median time to CR and CRh (n=15)7: 9.2 months
CR+CRh: 28%
PR: 28%
Clinical improvement: 15%
aMedian duration of follow-up was 11.6 months (95% CI: 9.9 to 16.3 months).
bClinical improvement is defined as having a response duration of ≥12 weeks and fulfillment of 1 or more of the nonhematologic and/or hematologic response criteria.8
CI=confidence interval; CR=complete remission; CRh=complete remission with partial hematologic recovery; ddPCR=droplet digital polymerase chain reaction; DOR=duration of response; ECNM=European Competence Network on Mastocytosis; ECOG PS=Eastern Cooperative Oncology Group Performance Status; IWG-MRT=International Working Group-Myeloproliferative Neoplasms Research and Treatment; NE=not estimable; PR=partial remission; WHO=World Health Organization.
Individual results may vary.
