EFFICACY
Patient portrayal
AYVAKIT was shown to reduce overall ISM symptom burden in the PIONEER clinical trial1
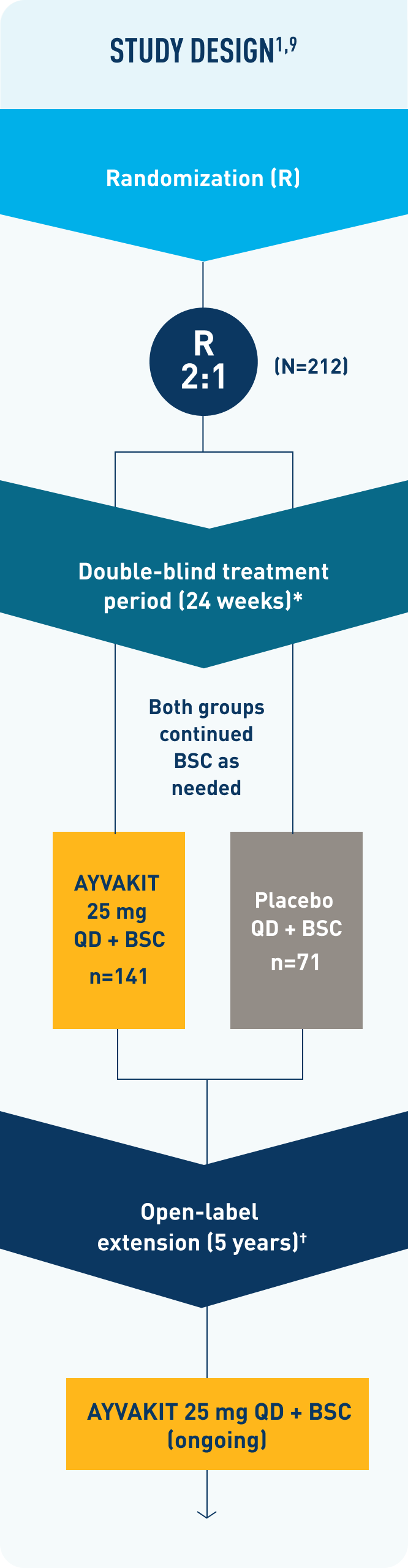
PIONEER (N=212) was a phase 2, multipart, randomized, placebo-controlled, double-blind study evaluating the efficacy and safety of AYVAKIT 25 mg + best supportive care (BSC) (n=141) vs placebo + BSC (n=71) over 24 weeks in adult patients (≥18 years) with a centrally confirmed ISM diagnosis per WHO criteria, and with moderate to severe ISM despite receiving at least 2 symptom-directed therapies. Patients were randomized 2:1 to receive once daily AYVAKIT 25 mg + BSC or placebo + BSC. Patients who completed the 24-week double-blind portion of the trial had the option to enter an open-label extension (OLE) treatment period for up to 5 years and receive once-daily AYVAKIT 25 mg + BSC.1,9
- Primary endpoint: Absolute mean change in Indolent Systemic Mastocytosis-Symptom Assessment Form (ISM-SAF) total symptom score (TSS) compared with placebo + BSC from baseline to Week 241
- Select key secondary endpoints: Proportion of patients achieving ≥50% reduction in serum tryptase levels; ≥50% reduction in KIT D816V VAF or undetectable‡; ≥50% reduction in bone marrow mast cells or no aggregates, all compared with placebo + BSC at Week 241
- Exploratory endpoints: Mean change in ISM-SAF individual symptom scores; mean change in most severe symptom score at Week 246,9
BSC medications allowed in the PIONEER study included anti-immunoglobulin E antibody (omalizumab), bisphosphonates, glucocorticoids, cromolyn sodium, H1 antihistamines, H2 antihistamines, leukotriene inhibitors, and proton pump inhibitors.9
*Data cutoff was June 23, 2022.
†OLE: The open-label study is evaluating the long-term efficacy and safety of AYVAKIT 25 mg + BSC for up to 5 years. All eligible patients either continued AYVAKIT 25 mg + BSC QD or switched from placebo + BSC to AYVAKIT 25 mg + BSC QD.
‡In peripheral blood.
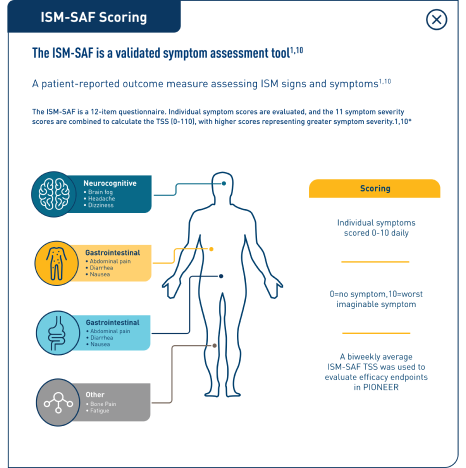
The ISM-SAF is a validated symptom assessment tool1,12
The ISM-SAF is a validated patient-reported outcome measure assessing ISM signs and symptoms using a 12-item questionnaire. Individual symptom scores are evaluated, and the 11 symptom severity scores are combined to calculate the TSS (0-110), with higher scores representing greater symptom severity.1,12
AYVAKIT demonstrated a statistically significant improvement in ISM-SAF TSS at 24 weeks1
| Week 24 | AYVAKIT + BSC (N=141) | Placebo + BSC (N=71) | 2-sided P value |
| Absolute mean change in the ISM-SAF TSS (95% CI) | -15.33 (-18.36, -12.31) | -9.64 (-13.61, -5.68) | 0.012 |
ITT analysis: Markov chain Monte Carlo simulation was used to impute the missing values at baseline or Week 24.
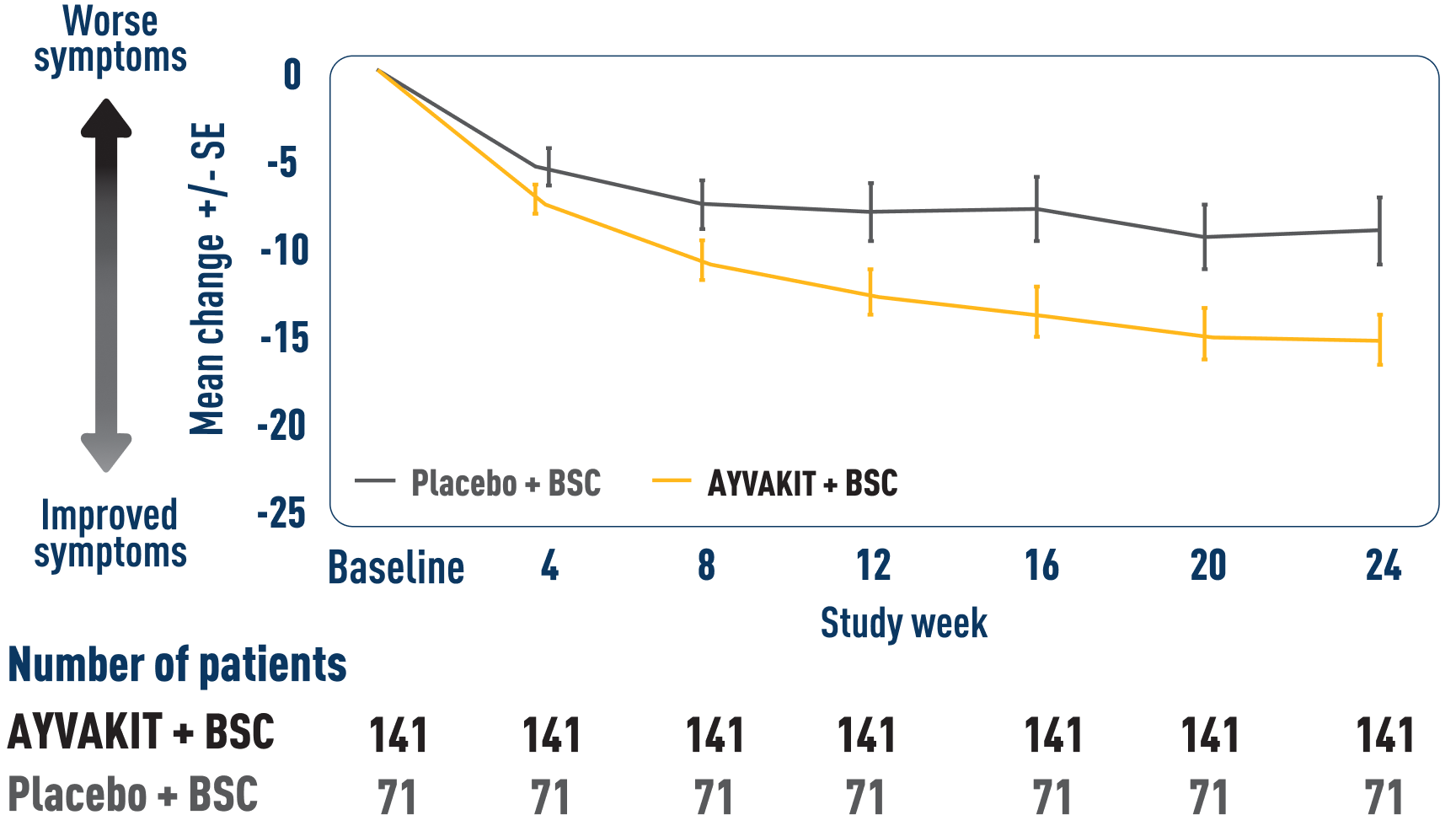
ISM-SAF TSS OVER TIME9
ITT analysis: Markov chain Monte Carlo simulation was used to impute the missing values.
LIMITATIONS: Mean change in TSS at all time points except Week 24 were prespecified, nonranked endpoints and were not adjusted for multiplicity. Therefore, treatment differences at these time points cannot be regarded as statistically significant and results should be interpreted with caution.
ITT=intention to treat.
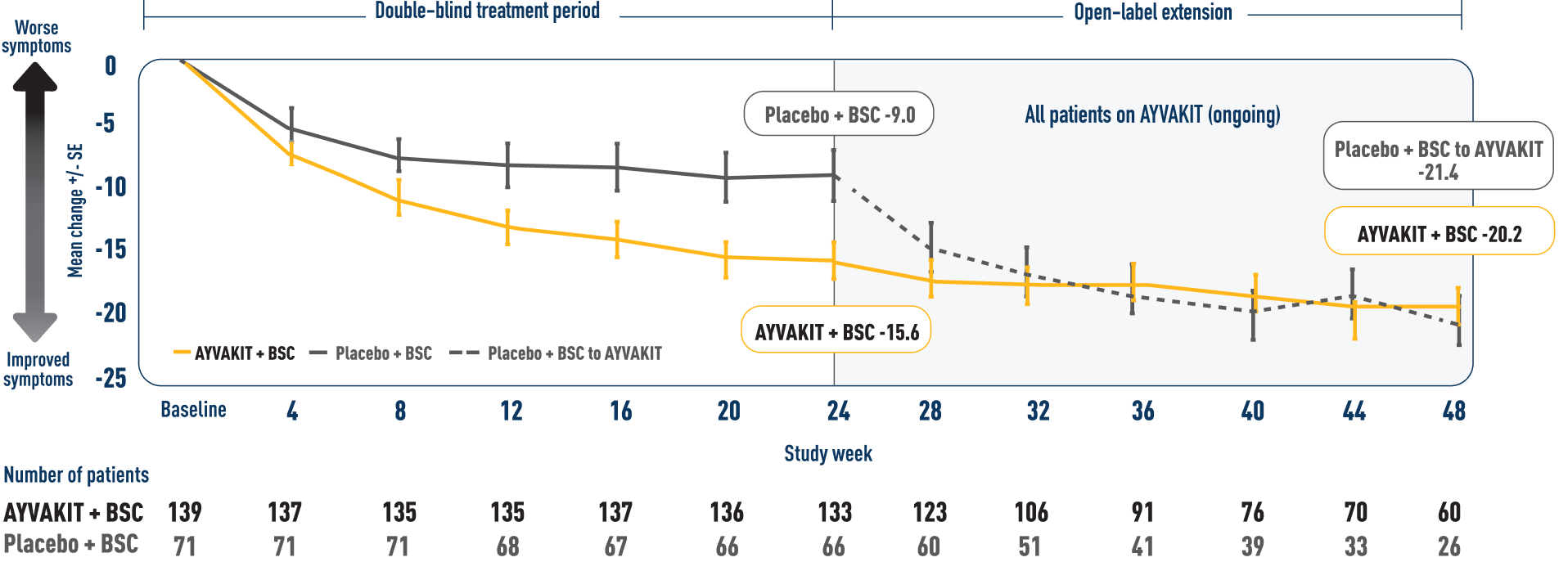
ISM-SAF Total Symptom Score through 48 weeks9
MEAN CHANGE IN ISM-SAF TOTAL SYMPTOM SCORE THROUGH 48 WEEKS9
ITT analysis: Patients with use of high-dose steroids (3 patients treated with AYVAKIT and 1 in the placebo group) were included in this analysis who were not included in the primary analysis. Missing visit data were excluded from calculations for that visit.
- In an interim analysis, a decrease in TSS was observed for patients who continued on AYVAKIT in an open-label extension through 48 weeks9
LIMITATIONS: Mean change in TSS at all time points except Week 24 were prespecified, nonranked endpoints and were not adjusted for multiplicity. Therefore, treatment differences at these time points cannot be regarded as statistically significant and results should be interpreted with caution and conclusions cannot be drawn. In an open-label extension, there is a potential for enrichment of the long-term data in the remaining patient populations since patients who were unable to tolerate or do not respond to the drug often drop out.
Reduced individual symptom scores across all symptoms were observed at Week 246
MEAN CHANGE FROM BASELINE AT 24 WEEKS BY ISM-SAF INDIVIDUAL SYMPTOM SCORE6
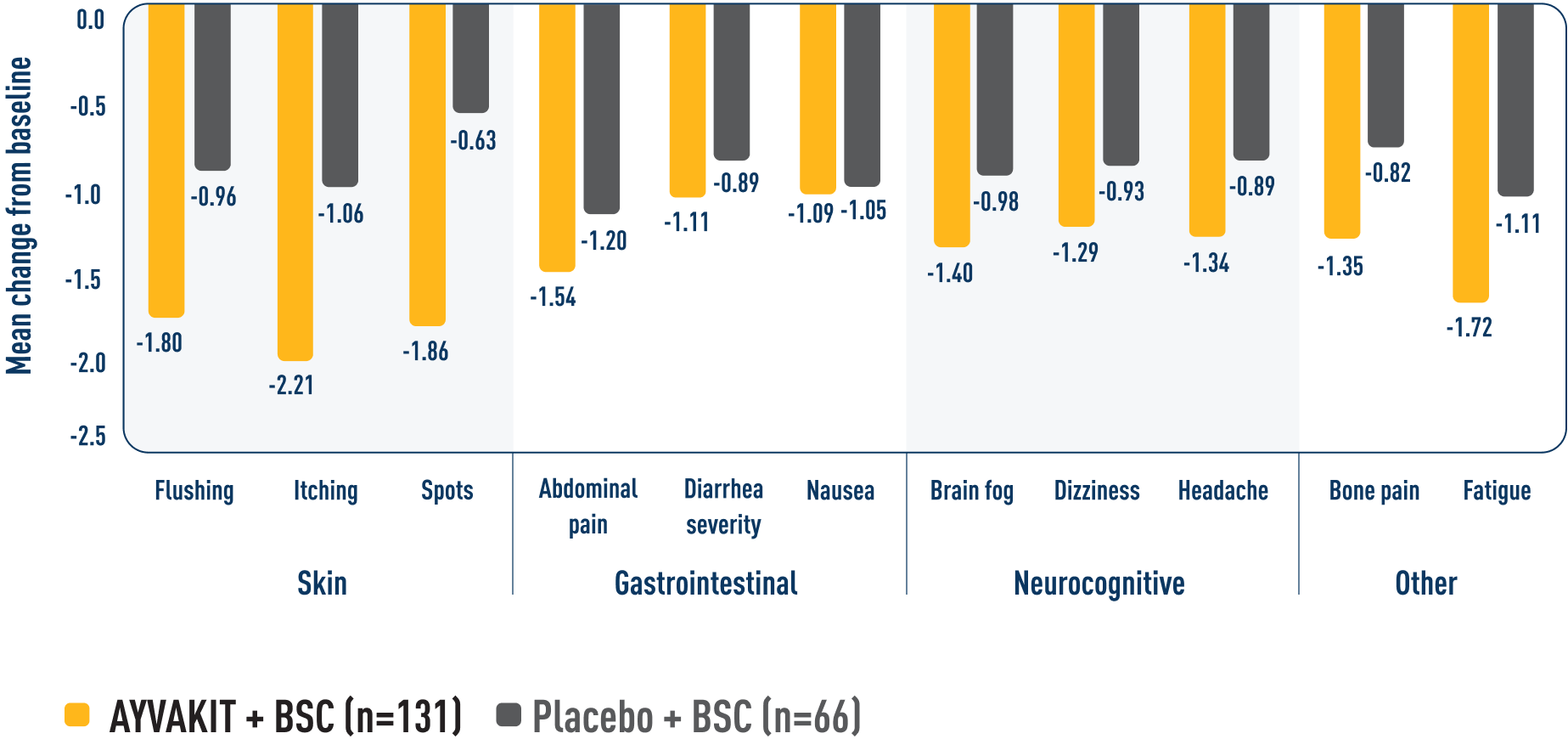
Reductions were observed in patients' most severe symptom, defined as the symptom with the highest score at baseline.9
ITT analysis: In this analysis, if a patient was missing more than 7 days of the score between baseline score and Week 2 score, it was considered as missing for the patient. If a patient missed more than 7 days of the score from the 14-day period for calculating the Week 24 score, then the Week 24 score was considered as missing.
LIMITATIONS: Individual components in a descriptive exploratory analysis were prespecified, nonranked endpoints, not adjusted for multiplicity, and not powered. Therefore, data should be interpreted with caution, conclusions cannot be drawn, and treatment differences cannot be regarded as statistically significant.
Significantly more patients treated with AYVAKIT had reductions in objective measures of mast cell burden at 24 weeks1
More than half of the evaluated patients in the AYVAKIT + symptom-directed therapies arm experienced ≥50% reductions in serum tryptase, KIT D816V VAF, and BM MC levels.1
PROPORTION OF PATIENTS ACHIEVING ≥50% REDUCTION IN OBJECTIVE MEASURES OF MAST CELL BURDEN AT 24 WEEKS1
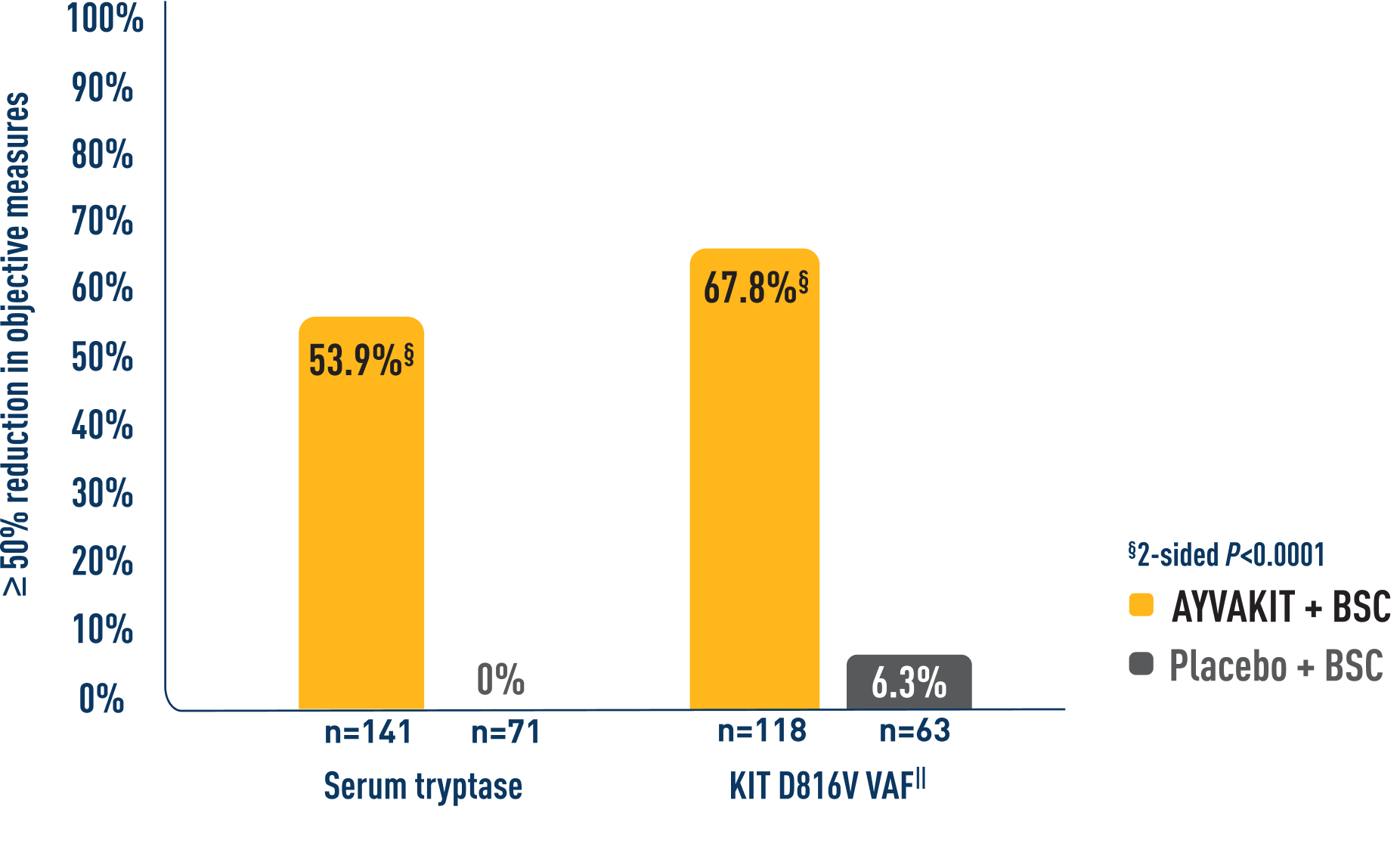
- BM MC levels: 52.8% of 106 patients receiving AYVAKIT + symptom-directed therapies achieved ≥50% reduction in BM MCs vs 22.8% of 57 patients receiving placebo + symptom-directed therapies (2-sided P<0.0001)1¶
ITT analysis: For patients with high-dose steroid use within 7 days before Week 24, or greater than 14 consecutive days at any point from baseline to Week 24, the Week 24 score was set to missing.
‖Percent of patients with ≥50% reduction in peripheral blood KIT D816V VAF or undetectable.
¶Percent of patients with ≥50% reduction in BM MCs or no aggregates.
BM=bone marrow; BSC=best supportive care; MC=mast cell; QD=every day; VAF=variant allele fraction.